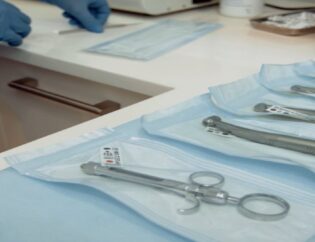
When it is a contamination-free environment, the packaging becomes mission-critical. The right packaging solutions lock in sterility from fill line to finished dose, shield components during harsh autoclave cycles, and satisfy FDA auditors without forcing extra steps.
If your current films turn brittle after steam or leave fibers in an ISO 5 zone, keep reading—this post shows you how to upgrade, what to look for, and where to get dependable performance.
Why Sterile Packaging Matters Right Now

Clean-room throughput is climbing, yet regulatory scrutiny has never been tougher. New guidance on particulate counts and extractables means any cleanroom materials must pass stricter tests.
At the same time, advanced medical device packaging designs rely on thin but rugged barrier layers to speed heat transfer during sterilization. Getting both durability and purity in one film is no longer optional—it’s essential for uptime and product release.
The Hidden Costs of Packaging Failure
A torn pouch or flaked film looks like a minor defect—until it halts production. Failed seals, pinholes, or fibers can lead to:
✓unplanned line shutdowns for re-cleaning
✓batch re-validation and costly hold times
✓warning letters for inadequate FDA-compliant packaging
Worse, compromised wrap invites biological ingress that a standard sterility assurance level (SAL 10-6) can’t forgive. In short, weak materials turn routine sterilization into a profit-eating fire drill.
Choosing the Right Packaging Solutions for Your Cleanroom
Material Integrity Under SterilizationLook for autoclave-resistant films that stay pliable at 134 °C, resist radiation yellowing, and shrug off plasma or ETO cycles. Multi-layer nylon structures excel here, offering low moisture uptake and high burst strength even after repeated steam exposure. |
Particulate Control & Cleanroom CompatibilityTrue ISO 5 films are extruded, slit, and bagged in Class 100 suites, then double-bagged to prevent inbound debris. Surface energy should be low enough to repel shed skin cells yet high enough to accept label inks without smudging—no compromises. |
Regulatory ReadinessDocumentation is more than a checkbox. Choose suppliers who provide full traceability, USP ⟨661.1⟩ and ⟨87⟩ test data, and certificates for sterile packaging for pharmaceuticals. When the FDA or an EU inspector walks in, you can hand over a single technical file—not a binder of gap analyses. |
Real-World Impact—Cleanroom Materials in Action
A biotech fill-finish team recently replaced conventional PE bags with high-performance nylon packaging built for gamma and steam. The switch cut tear-related rejects by 78 %, trimmed autoclave cycle time by 12 % thanks to faster heat transfer, and eliminated week-long hold periods for reinspection.
Another device assembler credits nylon liners with doubling the shelf life of moisture-sensitive reagents while meeting ISO 11607 burst tests on the first pass. Results like these turn packaging from overhead into measurable ROI.
Resources to Strengthen Your Packaging Program
✓Material selector chart – Compare barrier, flex-crack, and puncture scores side-by-side.
✓Sterilization cycle calculator – Estimate dwell time reductions with autoclave-resistant films.
✓Validation checklist – A ready-to-use template that aligns with CFR 21 Part 211 and ISO 13485.
✓Expert hotline – Quick access to process engineers who can troubleshoot seal temperature, dwell, and cooling profiles before deviations hit your batch record.
Frequently Asked Questions
Q: Can one film handle both gamma and steam?
Yes—look for multi-layer nylon/poly structures validated to 25 kGy and 134 °C.
Q: How do I prove particulate suitability?
Request 0.5 µm particle data and out-of-bag test results for Class 100 limits.
Q: Is nylon recyclable?
Many grades qualify for mechanical recycling under resin code 7; ask your waste provider.
Ready For Packaging That Performs?
When you need a film that resists autoclaves, guards purity and breezes through audits, turn to M&Q Packaging. Their cleanroom-manufactured nylon products deliver the sterile barrier, documentation, and supply continuity you require—without surprises.
Explore datasheets, request samples, or tap their engineers for one-on-one support, and keep your sterility assurance truly airtight.