Disinfection and sterilization are critical practices in healthcare facilities to limit the transmission of infectious pathogens. Clinicians are responsible for knowing when to clean, disinfect, or sterilize patient-care items based on the intended use, manufacturers’ recommendations, and guidelines. Keep reading to learn about the fundamental aspects clinicians should know about disinfection and sterilization in healthcare facilities.
How to clean patient-care devices
Proper cleaning, disinfection, and sterilization of patient-care devices is crucial in ensuring the safety of patients and healthcare workers. Here are some best practices to follow to help guarantee the effectiveness of the cleaning procedure:
- For better quality control, it’s recommended to perform cleaning, disinfection, and sterilization of patient-care devices in a central processing department within hospitals. This ensures optimal hygiene standards and makes maintaining quality much easier.
- Clinicians should thoroughly clean patient-care items with water and detergent or with water and enzymatic cleaners and remove visible organic residue (e.g., blood and tissue) and inorganic salts during cleaning.
- Immediately clean medical devices after use, either through manual or mechanical cleaning, to prevent soiled materials from drying or baking onto the instruments. This makes the removal process more challenging and the sterilization process less effective.
- If using an automatic washer/disinfector, follow the manufacturer’s recommendations and ensure that the detergents or enzymatic cleaners are compatible with the metals and other materials used in medical instruments. Take extra precautions when rinsing to adequately remove cleaning residues.
- Carefully Inspect equipment surfaces for breaks in integrity that would impede proper sterilization. Discard faulty equipment that can no longer be cleaned or disinfected.
Proper methods of sterilization
Although there are various methods for sterilization, such as steam, dry heat, chemical, and radiation sterilization, steam is the preferred method for many medical and surgical instruments. The highest level of sterilization requires following strict guidelines and parameters, including sterilization times, temperatures, and other operating parameters. Make sure to carefully read and follow the manufacturer’s instructions for the sterilizer, container, and wrap used for the equipment. Failure to comply with these guidelines can lead to ineffective sterilization and pose a serious risk to staff or patient safety.
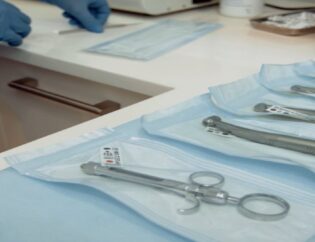
How to choose the right sterilization packaging
Quality sterilization packaging is the best method to shield medical devices from external contaminants such as microorganisms, moisture, and pathogens. Additionally, highly durable, heat-resistant nylon packaging can protect against punctures and tears. Packaging must be designed for common sterilization methods, including steam and dry heat autoclaves and gamma radiation sterilization. Lastly, ensure sterilization packaging products are made from FDA-approved grade A resins, meet class 100 standards, and are registered as an FDS 510(k) Class II Medical Device.
How to store sterile items
- Ensure packaging is uncompromised, and store items in a well-ventilated area protected from dust, moisture, insects, and temperature and humidity extremes.
- If the packaging is compromised (e.g., torn, wet, or punctured), reprocess the pack before use.
- Label sterilized items using the corresponding load number indicating the sterilizer used, the date of sterilization, and the expiration date.
- Use a quality control program for sterilized items that include the following: a sterilizer maintenance contract with service records; a system of process monitoring; air-removal testing for pre-vacuum steam sterilizers; visual inspection of packaging materials; and traceability of load contents.
High-quality sterilization packaging you can count on.
At M&Q, our nylon sterilization packaging solutions are designed for common sterilization methods, can withstand extreme temperatures, meet class 100 standards, and are registered as an FDS 510(k) Class II Medical Device. Learn more about our high-performing packaging here.
Disinfection and Sterilization Guidelines | Guidelines Library | Infection Control | CDC. www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html#anchor_1555614967.